Molded Products manufacturers various configurations of non-sterile lines and connectors that can be used in the hemodialysis process. The connectors are able to connect to the blood port side of a hemodialyzer and be installed into tubing giving the technician the ability to connect directly to the dialyzer for pre-rinsing, testing, or reprocessing.
Specific products include:

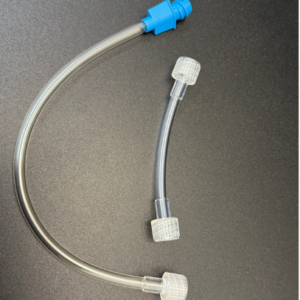
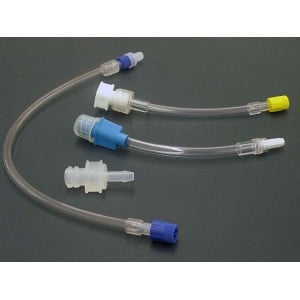



All Molded Products, Inc., products are registered with the U.S. Food and Drug Administration (FDA) as Class I and Class II medical devices. Devices are classified according to the degree of difficulty or level of control necessary to provide reasonable assurance of its safety and effectiveness. Devices must be sold under conditions specified by regulatory controls.